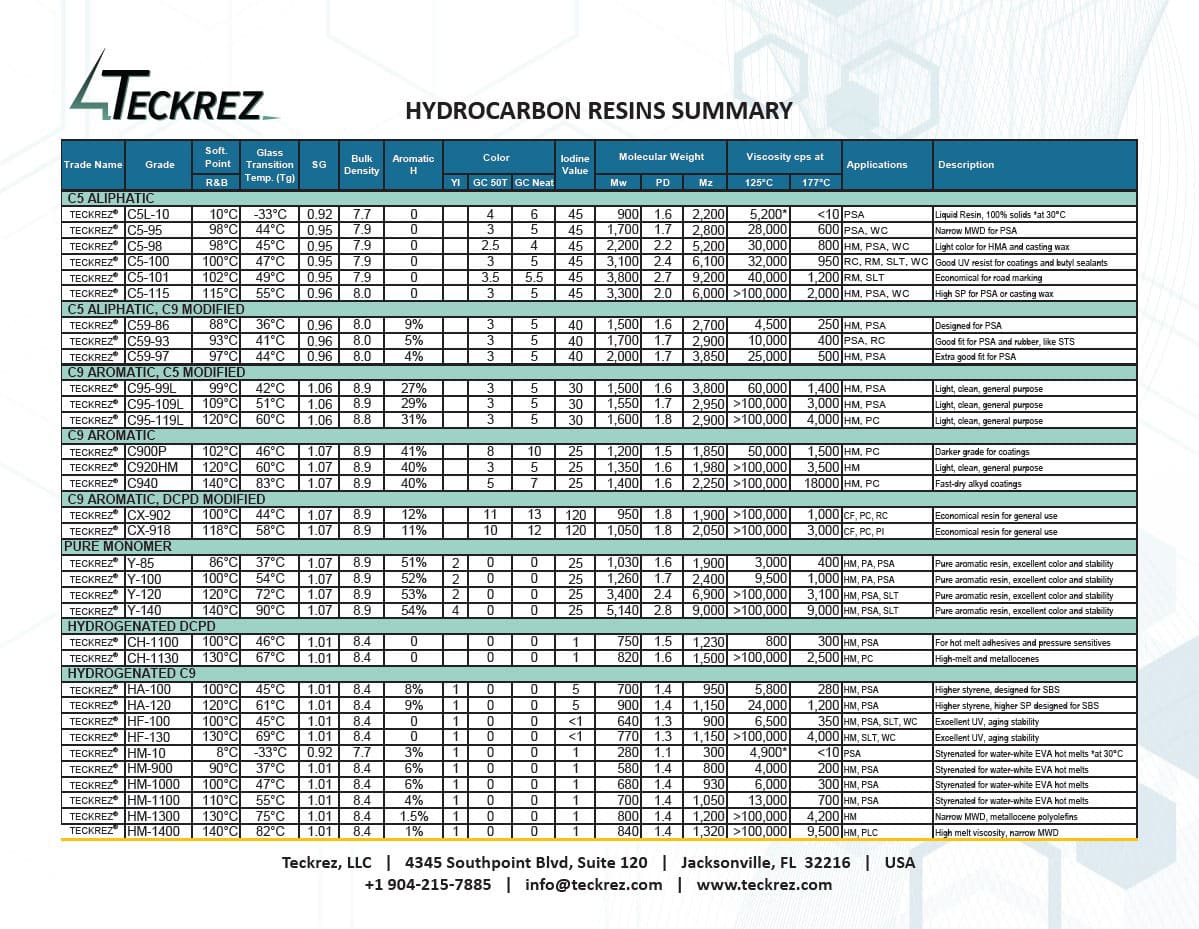
TECKREZ® Hydrocarbon Resins

Looking for Escorez Alternatives?
Teckrez® offers Aliphatic Resins, Aromatically Modified Aliphatic Resins, and Hydrogenated Resins, continuing the trusted properties and performance of Escorez® tackifiers.
Looking for Wingtack Alternatives?
Teckrez® offers tackifier resins from C5 and C5/C9 feedstocks, continuing the trusted properties and performance of Wingtack® tackifiers.
C5 Aliphatic Resins
C5 aliphatic resins are a type of hydrocarbon resin that is derived from the cracking of petroleum fractions such as naphtha or gas oils. These resins are typically composed of five-carbon (C5) aliphatic monomers, such as piperylene and isoprene, which are polymerized to form a solid, thermoplastic material.
C5/C9 AND C9/C5 Resins
C9/C5 aromatic/aliphatic resins, also known as hybrid resins, are a type of hydrocarbon resin that combines both aromatic and aliphatic monomers in their production. The aromatic monomers typically used are derived from the distillation of coal tar or petroleum, such as styrene, vinyltoluene, and indene. The aliphatic monomers are usually C5 fractions obtained from the cracking of petroleum.
The chemistry of C9/C5 aromatic/aliphatic resins is characterized by the combination of properties from both aromatic and aliphatic hydrocarbon resins. The presence of aromatic monomers gives the resin improved heat resistance, UV resistance, and color stability, while the aliphatic monomers contribute to flexibility, low odor, and improved tackiness.
C9 Aromatic Resins
C9 aromatic resins are a type of hydrocarbon resin that are derived from the distillation of coal tar or petroleum. They are composed of aromatic monomers, such as styrene, vinyltoluene, and indene, which are polymerized to form a solid, thermoplastic material.
The chemistry of C9 aromatic resins is characterized by their high degree of unsaturation and molecular weight, which gives them their unique properties. Specifically, C9 aromatic resins have a high content of double bonds and a molecular weight range of around 500-2000 g/mol.
Pure Monomer Resins
Pure monomer resins, also known as alpha methyl styrene resins, are a type of hydrocarbon resin that is derived from the polymerization of alpha-methylstyrene (AMS) monomer. AMS is a derivative of styrene that contains a methyl group on the alpha-carbon, which gives it unique properties compared to regular styrene.
The chemistry of pure monomer resins is characterized by the polymerization of AMS monomer through a free radical mechanism. During the polymerization process, the double bond on the styrenic ring of AMS is broken, and the monomers are joined together to form a polymer chain.
Pure monomer resins have a unique molecular structure that gives them unique properties compared to other hydrocarbon resins. The presence of the methyl group on the alpha-carbon of AMS monomer gives the resulting resin improved heat resistance, chemical resistance, and electrical insulation properties.
Hydrogenated Hydrocarbon Resins
Hydrogenated hydrocarbon resins are a type of hydrocarbon resin that has undergone a hydrogenation process. The result is a saturated hydrocarbon structure with improved stability, color, and odor properties.
The chemistry of hydrogenated hydrocarbon resins is characterized by the addition of hydrogen to the carbon-carbon double bonds in the molecular structure of the starting material. This is typically accomplished through a catalytic hydrogenation process, in which hydrogen gas is reacted with the resin in the presence of a catalyst, such as nickel or palladium.
The hydrogenation decreases molecular weight, improves thermal stability, and reduces its reactivity towards oxidation and UV light.
Comments/Applications: Designed for PSA
Comments/Applications: Good fit for PSA and rubber, like STS
Comments/Applications: Extra good fit for PSA
Comments/Applications: Light, clean, general purpose
Comments/Applications: Light, clean, general purpose
Comments/Applications: Light, clean, general purpose
Comments/Applications: Darker grade for coatings
Comments/Applications: Light, clean, general purpose
Comments/Applications: Fast-dry alkyd coatings
Comments/Applications: Economical resin for general use
Comments/Applications: Economical resin for general use
Comments/Applications: Pure aromatic resin, excellent color and stability
Comments/Applications: Pure aromatic resin, excellent color and stability
Comments/Applications: Pure aromatic resin, excellent color and stability
Comments/Applications: Pure aromatic resin, excellent color and stability
Comments/Applications: For hot melt adhesives and pressure sensitives
Comments/Applications: High-melt and metallocenes
Comments/Applications: Higher styrene, designed for SBS
Comments/Applications: Higher styrene, higher SP designed for SBS
Comments/Applications: Excellent UV, aging stability
Comments/Applications: Excellent UV, aging stability
Comments/Applications: Styrenated for water-white EVA